Reference
Bone HG, Wagman RB, Brandi ML, et al. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5(7):513‐523. doi:10.1016/S2213-8587(17)30138-9
At a glance
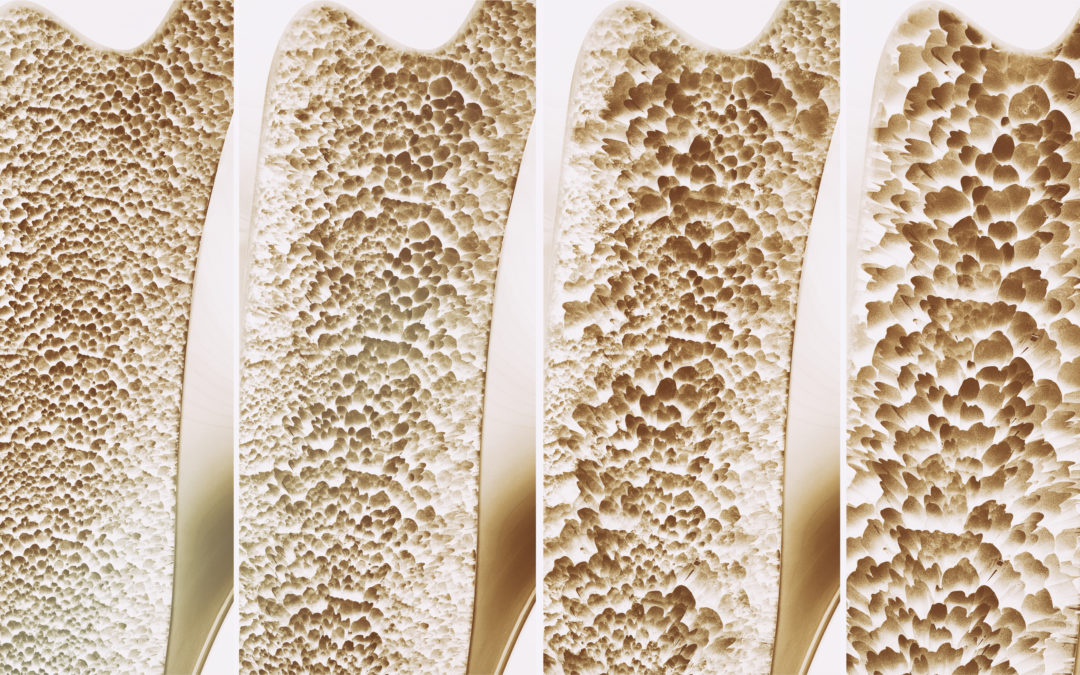
The present study is a 7-years open-label extension of the FREEDOM Trial, which demonstrated the effectiveness of Denosumab in reducing the rate of new osteoporotic fractures in post-menopausal women with osteoporosis. According to the result of the present paper, Denosumab treatment for up to 10 years was associated with low rates of adverse events, low fracture incidence and continued increases in BMD.
What is already known
Osteoporosis is a chronic condition characterized by low bone mass and microarchitectural deterioration of bone tissue, leading to bone fragility and increased fracture risk. After menopause, estrogen deficiency increases tissue exposure to RANK ligand (RANKL), resulting in increased bone resorption. Denosumab is a monoclonal antibody targeting RANKL; in the 3‑year FREEDOM trial, denosumab significantly increased bone mineral density (BMD), and reduced the rate of new vertebral, hip and non-vertebral fractures compared with placebo, in postmenopausal women with osteoporosis. Until the publication of the present paper, there were no data about long term safety and effectiveness of denosumab; this issue is particularly relevant, considering the chronic nature of osteoporosis and the need for long term treatment.
Design and Method
FREEDOM was a phase 3, multicentre, randomised, double-blind, placebo-controlled, 3-year trial done at 214 centres worldwide. Women with postmenopausal osteoporosis (with lumbar spine or total hip BMD T-score of less than −2,5 at either location but greater than –4,0 at both locations) aged 60–90 years old were enrolled and randomly assigned 1:1 to receive placebo or 60 mg denosumab subcutaneously every 6 months for 3 years.
During the 7-year extension, all participants received denosumab (60 mg) subcutaneously every 6 months and were instructed to take daily calcium and vitamin D; therefore, extension data represent up to 10 years of denosumab exposure for women who also received 3 years of denosumab in
FREEDOM (long-term group), and up to 7 years of denosumab exposure for women who received 3 years of placebo in FREEDOM (crossover group). 5928 out of 7808 women enrolled in the FREEDOM study were eligible for enrolment in the extension; of these, 4550 (77%) were enrolled (2343 long-term, 2207 crossover) and 2626 women (1343 long-term; 1283 crossover) completed the extension.
Patients were evaluated every 6 months and the following data were retrieved:
- Adverse events
- Clinical fracture information
- Measurements of the serum bone turnover markers
- BMD measurements during FREEDOM and at extension baseline and extension years 1, 2, 3, 5, and 7.
The primary objective of the extension was safety monitoring.
The main secondary endpoints were:
- Changes, and percent changes in BMD from FREEDOM baseline and extension baseline at all timepoints when BMD was collected;
- Incidence of vertebral fractures at months 24, 36, 60, and 84;
- Incidence of any non-vertebral fractures and hip fractures during the study.
- Changes in bone turnover markers from FREEDOM baseline and extension baseline.
Main Findings
The yearly exposure-adjusted incidence for all adverse events was stable throughout the study. During the extension, five subtrochanteric or diaphyseal femoral fractures occurred in the long-term group and four occurred in the crossover group; of these fractures, two were adjudicated as atypical (0.8 per 10000 participant-years). Moreover, 13 cases of osteonecrosis of the jaw were reported (5.2 per 10000 participant-years); the number of cases is very small, but the incidence is higher than that reported in the general population, being the individual risk affected by other factors: glucocorticoid use, maxillary or mandibular bone surgery, poor oral hygiene, chronic inflammation, diabetes mellitus, ill-fitting dentures, as well as other drugs.
In the long-term group, the annualised participant incidence of new vertebral fractures, non-vertebral fractures, and hip fractures during the extension remained similar to the incidence observed during the FREEDOM trial. In the crossover group, the annualised participant incidence of new vertebral fractures, non-vertebral fractures, and hip fractures was similar to that observed during the first 7 years of denosumab treatment in the long-term. Moreover, the relative risk of vertebral and non-vertebral fractures was lower than the one foreseeable on a virtual hypothetical cohort of placebo controls (virtual twin).
The median serum concentrations of bone turnover markers were reduced throughout the 7 years of the extension, while in the crossover group, median concentrations reduced rapidly after the initial administration of denosumab. Reductions in the crossover group were sustained throughout 7 years of treatment and were consistent with the results for the long-term group during the first 7 years of denosumab exposure.
Mean percentage changes in BMD from FREEDOM baseline (long-term group) and extension baseline (crossover group) to extension year 7 were significant. For both groups, the mean percentage changes from baseline in BMD were greater at each timepoint than those observed at the previous timepoint.
What’s new
In the FREEDOM extension trial, postmenopausal women with osteoporosis who were treated with denosumab for up to 10 years had an overall safety profile that remained consistent over time, with low fracture incidence (similar to rates observed during the FREEDOM trial) and continued gain in BMD. These findings distinguish denosumab among medications for long-term management of this chronic disease; thus, despite being still considered an alternative to bisphosphonate use, the profile of denosumab is unique and unlike bisphosphonates, routine interruption of Denosumab treatment is not recommended. Although the present paper was published three years ago, this topic is still hot: in fact, there are still concerns about the use of antiresorptive drugs which often impact on treatment adherence. In this paper, the authors demonstrated the long-term safety and effectiveness of denosumab.
Edited by Alessio Baricich and Daria Cuneo